|
More About the Madison Limestone
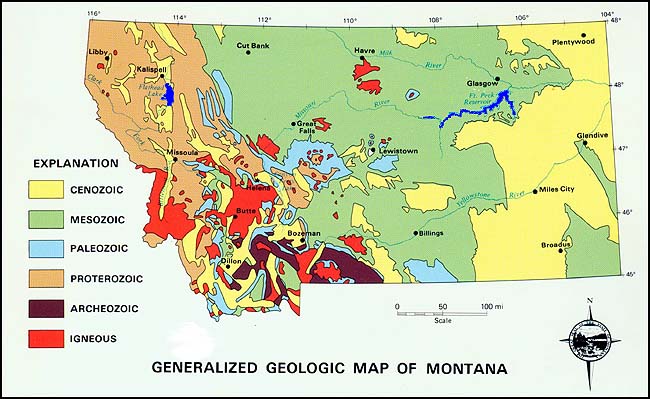 Map courtesy of Montana Bureau of Mines and Geology
The Madison limestone can be found at the surface or just beneath the soil in most of the light blue areas shown on the map above.
Exposed by plate tectonics . . .
A layer of reddish soil on top of the Madison Formation, called the Amsden Formation, indicates that the region was above sea level for a time after the limestone was deposited. Other marine formations above the Amsden tell of more recent times when Montana spent more time below sea level. In fact, throughout most of eastern Montana the Madison Formation is buried beneath layers deposited during the Mesozoic Era; the Age of the Dinosaurs (245 to 65 mill. yrs. ago). The primary reason the Madison can be seen in many of the places in central and western Montana is that plate tectonics made a mess of the layers during the late Mesozoic Era, especially in western Montana where it formed the Rocky Mountains. As the plates pushed together, in some places the Madison Formation was folded and/or faulted as it was raised toward the surface. As this was happening, or in the time since then, erosion removed layers overlying the limestone. In the central part of the state the folding was gentler, but in places like the Little Rockies, volcanism forced the limestone to the surface.
Toughest rock around . . .
In an article that he wrote for Montana Magazine several years ago, David Alt dubbed the Madison limestone as “Montana’s Rock of Ages”. Since limestone is made of the calcite, (the main ingredient in Tums and Rolaids), it is fairly soluble in rainwater*. Alt points out that limestones like the Madison haven’t faired so well in other places. For instance in the Appalachians rainfall has weathered away a similar formation so extensively that exposures are very hard to find. However, in Montana, our relatively dry climate (and slightly alkaline soils) makes the Madison and other limestones even more durable than granite.
*Rainwater is slightly acidic because it dissolves some CO2 from the air, forming carbonic acid.
|



