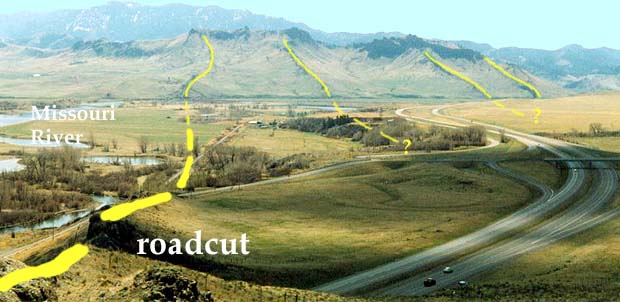
Below: Here is a photo taken from
the opposite side of the roadcut, looking
toward the river. The yellow lines on the
bottom photo mark the location of other dikes
in the area. To find out more about these
dikes, click on the Hot Link
below.

From a book called The Age of the
Earth by G. Brent Dalrymple . . .
1. Potassium (K) is common in rocks and
there are several minerals in which it is the
principle element. A .00001 g sample of K
contains 150,00 trillion atoms, including over
17 trillion atoms of the radioactive K-40.
2. Argon (Ar) is an inert gas that does not
combine with other elements.
3. While the rock is molten the Ar formed by
the decay of K escapes the magma. After the
rock has solidified and cooled, the argon is
trapped within the crystal structure of the
minerals like bird in a cage, accumulating with
the passage of time.
4. K-40 (radioactive type, or isotope of
potassium) has a nearly ideal half-life of 1.25
billion years.
5. There are 339 isotopes of 84 different
natural elements, including 269 that area
stable and 70 that are radioactive (not
stable).
6. There are three kinds of potassium (3
isotopes). Of these 93.26 % are K-39, .0117
% are K-40, and 6.73 % are
K-41.
Here’s how it works . . .
A small amount (.0117%) of all K atoms are
the radioactive isotope known as K-40. As
years go by, the K-40 atoms gradually
undergo radioactive decay, changing into
Ar-40 atoms. Scientists know that it takes
1.25 billion years for half of the K-40 to change
into Ar-40. This length of time is called the
“half-life,” and it is different for other
radioactive isotopes. For example, carbon-14,
which is found in living things (and dead
things) has a half-life of only 5,730 years. If
geologists find that an igneous rock has a K to
Ar ratio of 1 to 1, they would conclude that the
magma hardened 1.25 billion years ago. If
the ratio is 1:3, then two half-lives have gone
by . . . the rock is 2.5 billion years old.
|



