|
Salinity is an Important Aspect of Physical Oceanography
Think "big picture" here . . .
One of the most important "big ideas" in Earth Science is that everything is connected. A great example of this is the long list of factors that influence climate, including the position of continents and mountains (geology), ocean currents (oceanography), changes in the shape of Earth's orbit (astronomy), volcanism (geology), and many more. Astronomy, geology, oceanography, hydrology, and meteorology are are all interconnected in fascinating ways. Even though Montana may be hundreds of miles from the nearest ocean, it is important for everyone to understand some basic principles of physical oceanography in order to understand important Earth systems . . . and be able to look at our planet from a global perspective.
Parts per thousand? . . .
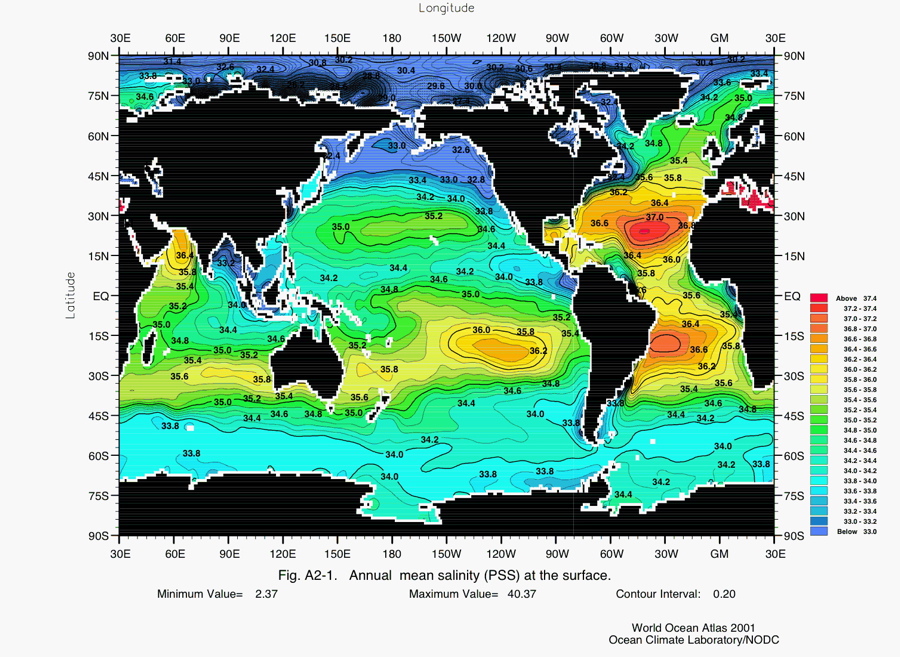
This map (above) uses colors to shows how salinity of surface waters in oceans varies throughout the world. Salinity, which is measure of how much salt is dissolved in the water, is expressed in parts per thousand (ppt). For example, surface waters along the equator between South America and Indonesia have salinities of about 34.6 ppt. This means that 1000 pounds of this salt-water, includes 965.4 pounds of water and 34.6 pounds of salt. Notice that equatorial waters tend to be saltier than those located 30 degrees north and 30 degrees south of the equator. The area along the equator generally gets much more rain than areas 30 degrees to the north or south because of the pattern of atmospheric circulation. The worlds great rain forests are near the equator (the Amazon, etc.), whereas some of the great deserts (the Sahara, etc.) are 30 degrees to the north or south. Observe how salty the Mediterranean Sea is compared to surface waters in the big oceans. The hot, dry climate of that region causes water to evaporate, leaving salts behind.
So what's up with those polar areas? . . .
As you look at the map, notice that surface waters in the polar areas are not as salty as other places, and also observe that surface waters in the Arctic less salty than those around Antarctica. One reason the polar waters are not as salty as those closer to the equator is simply because salt doesn't dissolve as easily in really cold water . . . and this is some REALLY cold water. As for the difference between, the Arctic and Antarctica, it has to do with precipitation and melting. Snowfall, and then the melting of snow and glaciers, which is more prevalent in the Arctic, adds freshwater to the Arctic Ocean. . . This dilutes the salt-water.
Salinity AND temperature affect density . . .
One of the driving forces in oceanic circulation (currents) is variations in densities of waters that originate in different source areas. Salinity is one of the factors that makes water more dense . . . Salty water is heavier than water that is not salty (as long as temperature is constant). A second factor that makes water more dense is temperature. Since the molecules in colder water are moving slower, they tend to be closer together. So if you had a beaker with 100 ml of hot water and one with 100 ml of cold water, the beaker of colder water will be heavier because it actually contains more water molecules. As it turns out the ocean water with the highest density is water that originates near Antarctica. There water can get down to a frigid -1.9 Celsius and have a salinity of 33.8 ppt. This water, which sinks to the ocean floor and moves northward, is appropriately named "Antarctic Bottom Water".
Watch a short YouTube video demonstration to see how salt affects the density of water: Salinity-Density Demonstration
The Day After Tomorrow (movie) . . .
To read about an interesting way that changes in salinity of water near Greenland may impact our climate, go to A Chilling Possibility. The NASA article discusses the theory upon which a popular movie (The Day After Tomorrow) was based.
Term: thermohaline, "the ocean conveyor belt", physical oceanography
|




